Debug (line 333 of ViewableData.php): SilverStripe\Blog\Model\BlogController created with a failover class of SilverStripe\Blog\Model\Blog
Debug (line 333 of ViewableData.php): SilverStripe\Blog\Model\BlogPostController created with a failover class of SilverStripe\Blog\Model\BlogPost
Debug (line 333 of ViewableData.php): SilverStripe\Assets\Storage\DBFile created with a failover class of SilverStripe\Assets\Image
Debug (line 333 of ViewableData.php): DNADesign\Elemental\Controllers\ElementController created with a failover class of DNADesign\Elemental\Models\ElementContent

Seep biogeochemistry & organic geochemistry
Storage of CO2 in geological formations depends on a combination of physical and chemical mechanisms such as physical trapping below caprocks or trapping by dissolution in groundwater. The most effective storage mechanism is the permanent mineralisation of CO2 by conversion into carbonate minerals (Benson et al., 2005). The relative trapping mechanisms contributions change through time after CO2 injection (Figure 1).
Basalts have an excellent storage potential because they are widespread around the world and because the mineralisation of injected CO2 is completed within a decade or less, reducing leakage risks to a minimum (Oelkers et al., 2023).
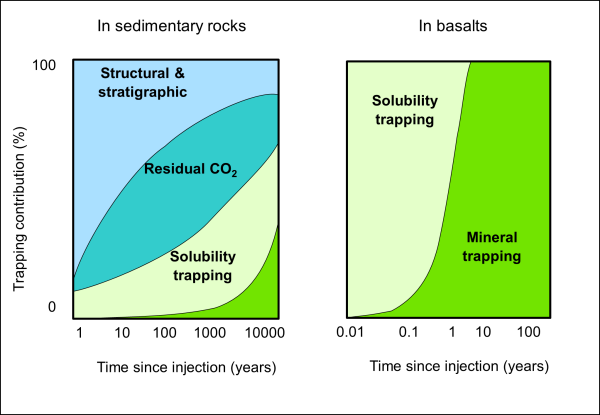
Figure 1: Relative contributions through time of different trapping mechanisms in sedimentary rocks (left) and basalts (right) (modified after Benson et al., 2005).
Carbonation occurs through the interaction of water-dissolved CO2 and formation minerals, which involves mineral dissolution followed by carbonate precipitation (Figure 2). The strongly acidic CO2-rich fluids (pH ~ 3 to 5) injected in the basalts promote the dissolution of silicates through the consumption of protons. The disaggregation of silicate minerals releases cations, which bind with carbonate anions as pH values rise due to the buffering effect of silicates dissolution (e.g., Oelkers and Gíslason, 2001; Heřmanská et al., 2021; Snaebjörnsdóttir et al., 2020).
The rapid mineralisation of injected CO2 releases considerable pressure on ensuring the high-quality and reliability of the caprock, which is a key constraint for the selection of adequate reservoir for storage in conventional settings (e.g., Kelemen et al., 2019). Nevertheless, whilst fractures in lava flows provide reactive surfaces for CO2 mineralisation, they still represent high permeability pathways for potential escape of CO2 until secondary carbonates “self-seal” the fractured reservoir.
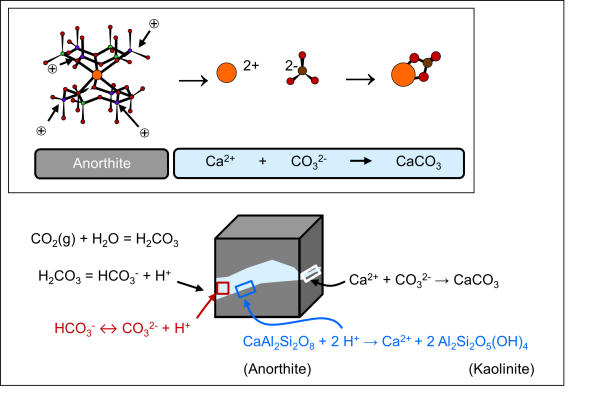
Figure 2: Schematic illustration of the related processes of dissolution of silicate minerals (here anorthite) by low pH in CO2-rich water and precipitation of carbonate minerals.
Understanding and predicting these mechanisms is the focus of our research on geochemical modelling of basalt carbonation (Nuzzo et al., 2024).
References:
Benson, S., Cook, P., et al. (2005). Underground geological storage, in IPCC special report on carbon dioxide capture and storage, Metz, B. O., Davidson, O., de Coninck, H. C., Loos, M., and Meyer, L. A. editors, Cambridge University Press, 195-276.
Heřmanská, M., Voight, M. J., Marieni, C., Declercq, J., Oelkers, E. H. (2021). A comprehensive and consistent mineral dissolution rate database: Part I: Primary silicate minerals and glasses. Chemical Geology 597, 120807.
Kelemen, P., et al. (2019). An overview of the status and challenges of CO2 storage in minerals and geological formations. Frontiers in Climate Review, November 2019, Vol. 1, Article 9, 20pp.
Nuzzo, M., Gupta, S., Burwicz-Galerne, E., and Galerne, C. (2024). A new multiphysics protocol to constrain subsurface CO2 plume migration for optimised carbon emissions monitoring in CCS. AAPG 8th Conjugate Margins Conference, Geo-energy session, May 2024.