Debug (line 333 of ViewableData.php): SilverStripe\Blog\Model\BlogController created with a failover class of SilverStripe\Blog\Model\Blog
Debug (line 333 of ViewableData.php): SilverStripe\Blog\Model\BlogPostController created with a failover class of SilverStripe\Blog\Model\BlogPost
Debug (line 333 of ViewableData.php): SilverStripe\Assets\Storage\DBFile created with a failover class of SilverStripe\Assets\Image
Debug (line 333 of ViewableData.php): DNADesign\Elemental\Controllers\ElementController created with a failover class of DNADesign\Elemental\Models\ElementContent

Senior Petroleum Geochemist / Basin Modeller
By Tiago Cunha & Marianne Nuzzo
MARUM - Centre for Marine Environmental Sciences, University of Bremen, Germany
Javier García-Pintado & Marta Pérez-Gussinyé
We introduced the usage of a box-size numerical model of miscible multiphase flow, with explicit advection and diffusion in both the liquid and gas phases, to model the migration and trapping of hydrogen in sedimentary basins in a previous communication (LINK - https://igiltd.com/news/modelling-hydrogen-migration-and-trapping-phase-1). Here, we demonstrate the usage of the box model to test the consumption-preservation of hydrogen in underground conditions of pressure and temperature that might be favorable –or not– for the development of microbial communities.
Microbial consumption of H2 (“hydrogenotrophy”) is the dominant process under anoxic subsurface conditions and is likely to be enhanced in H2 reservoir formations (e.g. Thaysen et al., 2021). In porous media, microbes are embedded in a biofilms that cover minerals/sediment grains (Figure 1). Migrating from porewater, H2 travels diffusively within the biofilm, and a variety of bacteria and archaea microbes are involved in its consumption, with the three most significant metabolic processes being: 1) hydrogenotrophic methanogenesis (CO2 + 4 H2 → CH4 + 4 H2O); 2) sulphate reduction (SO42- + 5 H2 → H2S + 4 H2O); and 3) acetogenesis (CO2 + 2H2 → CH3COOH) (e.g., Derya et al., 2015). Unless H2 is sufficiently abundant, they compete for the H2 substrate and the microbes able to live at the lowest H2 concentration thresholds outcompete the other groups. Here we report on initial tests of multiphase H2 migration with the consideration of microbial consumption of dissolved H2, where the kinetic reaction of hydrogenotrophic methanogenesis is applied in a simplified form to the bulk representative element volume, without explicit consideration of the dynamics of (and within) the biofilm layer.
To simulate microbial growth, we follow the general assumption that growth rates are reasonably well reproduced by Monod kinetics (e.g., Thaysen et al., 2021):
where μ is the specific growth rate, μmax the maximum specific growth rate, [H2] the H2(aq) concentration, and Ks the half-saturation constant. The above equation implies that for this exercise we ignore the concentration of CO2, assuming it is always available, and non-limiting in the reaction.
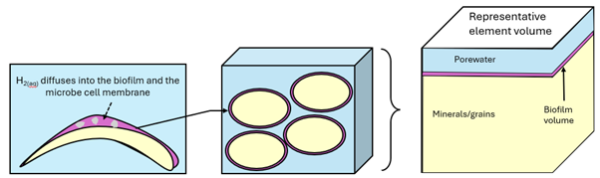
Figure 1. Illustration of the coupled pore-scale model for methanogenic microbial activity in underground hydrogen storage (after Ebigbo, et al., 2013).
The microbial biomass at any given time is represented by an exponential relationship controlled by the growth rate:
where X(t) is the biomass at a given time (t), X(0) the initial biomass and μ the specific growth rate.
The rate of substrate depletion (microbial sink) is thus given by the general formula:
In the models we assume coupling between the rate of H2 consumption and microbial growth, depending on the concentration of dissolved H2, and include a dependence of microbial growth on temperature. The models are initiated with a low concentration of microbial biomass in the system.
We test the microbial sinks in our high resolution advection-diffusion fluid flow box model (Fig. 2a) for two surface P-T conditions: (1) at 100 m depth with a temperature at top of the model of 20°C (Fig. 2b); and (2) at 1000 m depth with a top temperature of 46°C (Fig. 2c). The models run for approximately 10 yr, after which they reach an approximate steady state, as a result of the high generation rates and the simple reservoir-seal parametrization in this model setup. The relatively high H2 input rates would represent, for example, an active serpentinization front or stimulated H2 generation in the neighbourhood of the domain, providing the input boundary conditions.
At near surface conditions (low pressure), the solubility of the gas in the water is low, the saturation of the gas phase is high, and the models predict the development of trapped gas layer controlled by the retarding capability of the lower permeability layers (Fig. 2b). Due to the low H2 saturation in the liquid phase, the predicted microbial sink is also very small. The low temperatures in this model are also not optimal for a rapid microbial growth. In contrast, at higher pressure conditions, the models predict significantly higher H2 dissolution, with only a small fraction of the H2 in the gas phase, and significant microbial consumption.
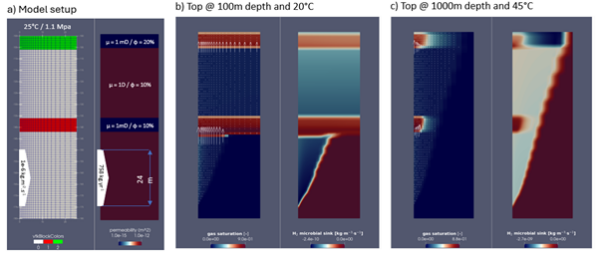
Fig. 2 – Box model predictions with varying P-T conditions for the development of microbial sinks. a) Setup of the high resolution box-model (see details in https://igiltd.com/news/modelling-hydrogen-migration-and-trapping-phase-1); b) Near surface conditions, with model top at 100 m (1.1MPa) depth and 20ºC. c) Top model at 1000m (10.1 MPa) depth and 45ºC.
REFERENCES
Derya, O., Hyunsoo, N., Lever M.A., Kasper, K. U., Jørgensen, B.B., Plugge, C.M., 2015. Methanogenic archaea and sulfate reducing bacteria co-cultured on acetate: teamwork or coexistence? Frontiers in Microbiology 6. www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2015.00492
Ebigbo, A., Golfier, F., Quintard, M., 2013. A coupled, pore-scale model for methanogenic microbial activity in underground hydrogen storage. Advances in Water Resources, Volume 61, 2013, Pages 74-85, ISSN 0309-1708, https://doi.org/10.1016/j.advwatres.2013.09.004.
García-Pintado, J., Pérez-Gussinyé, M., Abreu Cunha, T., Nuzzo, M., Lawrence, S., Jackson, O., Stocks, A., Barnicoat, A. & Hutchinson, I., 2023. Box-Size Numerical Modelling of Hydrogen Migration and Trapping: Testing the Geological Setting and Analogies with the Petroleum Systems. In Natural Hydrogen: A New Frontier for Energy Geoscience, 4-5 July 2023, Geological Society of London, London (UK). - Oral communication.
Garcia-Pintado, J., Perez-Gussinye, M. & Mezri, L., 2023. Spatio-temporal Dynamics of Hydrothermal Circulation over 10 million years of Ultraslow- Rifting and Spreading. GeoBerlin Geosciences Beyond Boundaries - Research, Society, Future, Berlin (Germany). - Oral communication.
Thaysen, E. M., McMahon, S., Strobel, G. S., Butler, I. B., Ngwenya, B. T., Heinemann, N., Wilkinson, M., Hassanpouryouzband, A., McDermott, C. I., Edlmann, K., 2021. Estimating microbial growth and hydrogen consumption in hydrogen storage in porous media. Renewable and Sustainable Energy Reviews 151(111481), ISSN 1364-0321, https://doi.org/10.1016/j.rser.2021.111481.